25I-NBOMe (2C-I-NBOMe, Cimbi-5, also shortened to "25I") is a psychedelic drug and derivative of the substituted phenethylamine psychedelic 2C-I. It was discovered in 2003 by chemist Ralf Heim at the Free University of Berlin, who published his findings in his PhD dissertation. The compound was subsequently investigated by a team at Purdue University led by David Nichols.
The carbon-11 labelled version of 25I-NBOMe, [11C]Cimbi-5, was synthesized and validated as a radiotracer for positron emission tomography (PET) in Copenhagen. Being the first 5-HT2A receptor full agonist PET radioligand, [11C]-CIMBI-5 shows promise as a more functional marker of these receptors, particularly in their high affinity states.
§Chemistry and structure

Like other 2C-X-NBOMe molecules, 25I-NBOMe is a derivative of the 2C family of phenethylamines described by Alexander Shulgin in his book PiHKAL. Specifically, 25I-NBOMe is an N-benzyl derivative of the phenethylamine molecule 2C-I, formed by adding a 2-methoxybenzyl (BnOMe) onto the nitrogen (N) of the phenethylamine backbone. This substitution significantly increases the potency of the molecule.
§Synthesis
25I-NBOMe can be synthesised from 2C-I and 2-methoxybenzaldehyde, via reductive alkylation. It can be done stepwise by first making the imine and then reducing the formed imine with sodium borohydride, or by direct reaction with sodium triacetoxyborohydride.
§Pharmacology
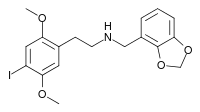
25I-NBOMe acts as a highly potent full agonist for the human 5-HT2A receptor, with a Ki of 0.044 nM, making it some sixteen times the potency of 2C-I itself at this receptor. A radiolabelled form of 25I-NBOMe can be used for mapping the distribution of 5-HT2A receptors in the brain. It is one of the only full agonists of the human 5-HT2A in existence.
25I-NBOMe induces a head-twitch response in mice which is blocked completely by a selective 5-HT2A antagonist, suggesting its psychedelic effects are mediated by 5-HT2A.This study suggested that 25I-NBOMe is approximately 14-fold more potent than 2C-I in-vivo.
While in-vitro studies showed that N-benzyl derivatives of 2C-I were significantly increased in potency compared to 2C-I, the N-benzyl derivatives of the related compound DOI were inactive.
Ki values of the following targets were greater than 500 nM: 5-HT1A, D3, H2, 5-HT1D, α1A adrenergic, δ opioid, serotonin uptake transporter, 5-HT5A, 5-HT1B, D2, 5-HT7, D1, 5-HT3, 5-HT1E, D5, muscarinic M1-M5, H3, and the dopamine uptake transporter.
A forensic standard of 25I-NBOMe is available, and the compound has been posted on the Forendex website of potential drugs of abuse.
§Recreational use
Although 25I-NBOMe was discovered in 2003, it did not emerge as a common recreational drug until 2010, when it was first sold by vendors specialising in the supply of research chemicals. In a slang context, the name of the compound is often shortened to “25Iâ€. According to a 2014 survery, 25I-NBOMe is the most frequently used of the NBOMe series. Case reports of 25I-NBOMe intoxication, with and without analytic confirmation of the drug in the body, are increasing in the medical literature.
25I-NBOMe is widely rumored to be orally inactive; however, oral efficacy has not been disproven and apparent overdoses have occurred via the oral route. Common routes of administration include sublingual, buccal, and nasal/intranasal. For sublingual and buccal administration, 25I-NBOMe is often applied to sheets of blotter paper of which small portions (tabs) are held in the mouth to allow absorption through the oral mucosa. There are reports of intravenous injection of 25I-NBOMe solution and smoking the drug in powdered form.
Due to its potency and much lower cost than so-called classical or traditional psychedelics, 25I-NBOMe blotters are sometimes misrepresented as, or mistaken for LSD blotters. It is dangerous to attempt to differentiate the two using sensory techniques (i.e. taste) but reagent testing (in particular ehrlich's reagent) can easily differentiate ergolines from 25I-NBOMe via colour change. Small quantities of 25I-NBOMe can provide a large numbers of doses. Vendors may import 25I-NBOMe in bulk and resell individual doses for considerable profit.
§Dosage
25I-NBOMe is potent, being active in sub-milligram doses. A common dose of the hydrochloride salt is 600â€"1,200 µg. The UK Advisory Council on the Misuse of Drugs states that a common dose is between 50 and 100 µg, although other sources indicate that these figures are incorrect; Erowid tentatively suggests that the threshold dosage for humans is 50â€"250 µg, with a light dose between 200â€"600 µg, a common dose at 500â€"800 µg, and a strong dose at 700â€"1500 µg. At this level of potency, it is not possible to accurately measure a single dose of 25I-NBOMe powder without an analytical balance, and attempting to do so may put the user at significant risk of overdose.
§Effects
25I-NBOMe effects usually last 6â€"10 hours if taken sublingually or buccally. When it is insufflated, effects usually last 4â€"6 hours. However, effects can last significantly longer depending on dosage; durations longer than 12 hours have been reported.
25I-NBOMe can also be vaporized and inhaled, this may cause significantly quicker effects and shorter duration as is expected from that route of administration. However, this route of administration is not recommended, unless using precise liquid measurement, due to the difficulties of measuring and handling substances active in the microgram range.
25I-NBOMe has similar effects to LSD, though users report more negative effects while under the influence and more risk of harm following use as compared to other classic psychedelics.
Case reports of seven British males who presented to an emergency room following analytically confirmed 25I-NBOMe intoxication suggest the following potential adverse effects: tachycardia (n = 7), hypertension (4), agitation (6), aggression, visual and auditory hallucinations (6), seizures (3), hyperpyrexia (3), clonus (2), elevated white cell count (2), elevated creatine kinase (7), metabolic acidosis (3), and acute kidney injury (1).
§Tolerance
Users of 25I-NBOMe have reported that it causes physiological tolerance that may last for around 2â€"4 weeks. This tolerance is described as diminishing the efficacy of subsequent doses of 25I-NBOMe, as well as interfering with the efficacy of other psychedelics. This kind of temporary, rapidly developed tolerance is a typical side effect of a number of other psychedelics, such as LSD. This tolerance may also affect the tolerance one has to a variety of other drugs.
§Toxicity and harm potential
Recreational use of 25I-NBOME carries significant risk of both pharmacological and behavioral toxicity. 25I-NBOMe is a relatively new substance, and little is known about its pharmacological risks or its interaction with other substances. The LD50 has not yet been determined. It is a highly potent serotonin agonist and, due to its psychedelic effects and ambiguous legal status, a designer drug with reports of recreational use beginning in 2010. Reports of deaths and significant injuries have been attributed to the use of 25I-NBOMe, prompting some governments to control its possession, production, and sale. The harm-reduction website Erowid states that 25I-NBOMe is extremely potent and should not be snorted as this method of administration “appears to have led to several deaths in the past year.†Several non-fatal overdoses requiring prolonged hospitalization have also been reported.
As of September 2013, 25I-NBOMe has reportedly led to at least six overdose deaths in the United States. In June 2012, two teens in Grand Forks, North Dakota and East Grand Forks, Minnesota fatally overdosed on a substance that was allegedly 25I-NBOMe, resulting in lengthy sentences for two of the parties involved and a Federal indictment against the Texas based online vendor. A 21-year-old man from Little Rock, Arkansas died in October 2012 after taking a liquid drop of the drug nasally at a music festival. He was reported to have consumed caffeinated alcoholic beverages for “several hours†beforehand. It is unclear what other drugs he may have consumed, as autopsies generally do not test for the presence of research chemicals. In January 2013, an 18 year-old in Scottsdale, Arizona, died after consuming 25I-NBOMe sold as LSD; a toxicology screening found no other drugs in the person's system. The drug is the suspected cause of death in another Scottsdale, Arizona, incident in April 2013. It is also cited in the death of a 21 year old woman in Aug 2013.
25I-NBOMe has been implicated in multiple deaths in Australia. In March 2012, a man in Australia died from injuries sustained by running into trees and power poles while intoxicated by 25I-NBOMe. A Sydney teenager jumped to his death on June 5, 2013. He reportedly jumped off a balcony thinking he could fly.
§Legal status
§Australia
25I-NBOMe was explicitly scheduled in Queensland (Australian) drug law in April 2012, and in New South Wales in October 2013, as were some related compounds such as 25B-NBOMe. The Australian federal government has no specific legislation concerning any of the N-benzyl phenethylamines.
§Israel
Israel banned 25I-NBOMe in 2013.
§Russia
Russia was the first country to pass specific regulations on the NBOME series. All drugs in the NBOMe series, including 25I-NBOMe, became illegal in Russia in October 2011.
§Sweden
25I-NBOMe was classified as a Schedule I substance in publication LVFS 2013:15 by the Medical Products Agency. The classification took effect on 1 Aug, 2013.
§United Kingdom
N-benzylated phenethylamines such as 25I-NBOMe were initially unaffected by the legal status of phenethylamine-class drugs. The British government issued a temporary class drug order on a list of emerging recreational drugs, including 25I-NBOMe, on 4 June 2013. The order, which took effect on 10 June 2013 and will last for up to twelve months, prohibits the production, import and sale of “the NBOMe and Benzofury groups of substancesâ€.
The UK Home Office announced that 25I-NBOMe would be made a class A drug on 10 June 2014 alongside every other N-benzyl phenethylamine.
§United States
On Nov 15, 2013, the DEA added 25I-NBOMe (and 25C-, and 25B-NBOMe) to Schedule I using their emergency scheduling powers, making those NBOMe compounds "temporarily" in Schedule I for 2 years.
§Romania
In 2011, Romania banned all psychoactive substances. For the substances that are not in any controlled substance table, only operations are not permitted without an explicit permit (e.g. selling)
§Serbia
25I-NBOMe was put on the list of controlled psychoactive substances in 2014, with the law coming into effect in 2015.
§See also
- 2CBCB-NBOMe (NBOMe-TCB-2)
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
- 25C-NBOMe (NBOMe-2CC)
- 25B-NBOMe (NBOMe-2CB)
- 25I-NBMD (NBMD-2CI)
- 25I-NBOH (NBOH-2CI)
- 25I-NBF (NBF-2CI)
- 5-MeO-NBpBrT
§References
§External links
- http://isomerdesign.com/PiHKAL/explore.php?domain=pk&id=5380
