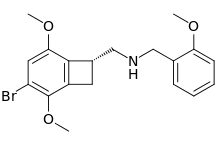
TCB-2 is a hallucinogen, discovered in 2006 by Thomas McLean, working in the lab of Prof. David Nichols at Purdue University where it was named 2C-BCB. It is a conformationally-restricted derivative of the phenethylamine 2C-B, also a hallucinogen, and acts as a potent agonist for the 5-HT2A and 5-HT2C receptors with a Ki of 0.26nM at the human 5-HT2A receptor. In drug-substitution experiments in rats, TCB-2 was found to be of similar potency to both LSD and Br-DFLY, ranking it among the most potent phenethylamine hallucinogens yet discovered. This high potency and selectivity has made TCB-2 useful for distinguishing 5-HT2A mediated responses from those produced by other similar receptors. TCB-2 has similar but not identical effects in animals to related phenethylamine hallucinogens such as DOI, and has been used for studying how the function of the 5HT2A receptor differs from that of other serotonin receptors in a number of animal models, such as studies of cocaine addiction and neuropathic pain.
The related benzocyclobutene analogs of mescaline, Tomscaline and Bromotomscaline also exhibit significantly higher potency than their parent compound. The N-BnOMe (N-ortho-methoxybenzyl) analogs of TCB-2, Tomscaline, and Bromotomscaline were also synthesized.
§See also

- 2CB-Ind
- Jimscaline
- 2CBCB-NBOMe
§References